
The decision between Optomap and dilation is not about comfort versus discomfort; it’s a strategic choice about the depth and breadth of diagnostic information you want to capture about your eye health.
- Wide-field imaging like Optomap excels at creating a permanent, wide-angle digital record of the retinal surface, crucial for monitoring peripheral risks and tracking changes over time.
- Traditional dilation provides a dynamic, three-dimensional view that is essential for assessing specific structures like the optic nerve in detail.
Recommendation: For comprehensive long-term health monitoring, leveraging both technologies—using Optomap as a baseline map and dilation for targeted deep dives—offers the highest diagnostic yield.
For many patients, the annual eye exam presents a familiar dilemma: endure the blurry vision and light sensitivity from dilation drops, or pay extra for a quick, comfortable Optomap scan? The debate is often framed around convenience. While the comfort of avoiding dilation is a significant benefit, this surface-level comparison misses the fundamental point. The real distinction lies in the type, breadth, and depth of diagnostic information each method provides. Choosing the right exam is less about your comfort today and more about your long-term health and the ability to detect disease before it impacts your vision.
This decision is not a simple “either/or” but a question of diagnostic strategy. Dilation, the long-standing gold standard, uses drops to widen the pupil, allowing the doctor a live, three-dimensional view into the eye. In contrast, ultra-widefield (UWF) retinal imaging, exemplified by the Optomap, uses scanning laser technology to capture a panoramic, high-resolution digital photograph of the retina’s surface in seconds. Understanding the unique strengths and inherent limitations of each approach is crucial for any patient proactive about their health.
This article moves beyond the comfort debate to provide a clear, objective analysis from a medical technology perspective. We will explore why certain pathologies hide in the retinal periphery, how to interpret the complex data from modern scans, and why the future of comprehensive eye care lies in the intelligent combination of these powerful tools. This is not just about seeing the back of your eye; it’s about understanding the story it tells.
To navigate this complex topic, we will break down the key technical and clinical differences. This guide will equip you to have a more informed conversation with your eye doctor about the examination strategy best suited to your personal health profile and risk factors.
Summary: Optomap or Dilation: Which Retinal Exam Is Right for You?
- Why Tears and Detachments Hide in the Far Periphery?
- How to Read the Layers of Your Retina on an OCT Image?
- Photo Documentation or Live Inspection: Why You Need Both?
- The Myth That Retinal Cameras Can Damage Your Vision With Bright Light
- When to Rush In: Sudden Flashes vs. Old Floaters
- How Retinal Imaging Replaces the Ophthalmoscope in Modern Exams?
- The Silent Retinal Issue That Standard Acuity Tests Miss in 1 out of 5 Patients
- Why Fluctuating Blood Sugar Causes Blurry Vision Even With New Glasses?
Why Tears and Detachments Hide in the Far Periphery?
The anatomy of the eye itself dictates where its most acute vulnerabilities lie. The central retina, including the macula, is robust and densely packed with photoreceptors for sharp, detailed vision. However, as the retina extends towards the edge of the eye, it becomes significantly thinner and more fragile. This area, known as the peripheral retina, is where the vitreous gel (the jelly-like substance filling the eye) is most strongly attached. As we age, this gel naturally liquefies and pulls away from the retina in a process called Posterior Vitreous Detachment (PVD). This pulling action, or traction, is most forceful in the periphery, making it the most common location for retinal tears to develop.
These peripheral tears are the precursors to retinal detachment, a medical emergency that can lead to permanent vision loss. Because they occur at the far edges, they often produce no symptoms in their early stages. A 2023 retrospective study confirmed the long-term risk, finding that 7.39% of patients develop delayed retinal tears within years after an acute PVD. This underscores the critical need for examination methods that provide a clear and wide view of these vulnerable zones.

This anatomical reality is precisely where ultra-widefield imaging like Optomap demonstrates its value. By capturing up to 200 degrees of the retina in a single image, it provides a comprehensive map of the periphery that can be difficult to achieve with traditional, smaller-field examination techniques. It allows for the detection of small, asymptomatic tears or areas of retinal thinning that might otherwise be missed, enabling early intervention. It also creates a permanent temporal record to monitor for subtle changes over time, a key advantage in managing long-term risk.
How to Read the Layers of Your Retina on an OCT Image?
While ultra-widefield imaging provides an expansive surface map of the retina, Optical Coherence Tomography (OCT) provides the crucial third dimension: depth. An OCT scan is akin to a cross-sectional MRI of the retina, using light waves to generate a high-resolution image of its distinct layers. For a patient, understanding the basics of an OCT image is empowering, as it transforms an abstract medical test into a tangible view of your ocular health. The scan reveals structures that are only microns thick, allowing for the detection of disease at a microscopic level long before it affects vision.
An OCT image is typically displayed in false color, where different colors represent varying degrees of light reflectivity from the retinal tissues. Clinicians are trained to identify the ten distinct layers, but for a patient, recognizing a few key landmarks offers significant insight. For instance, the Retinal Nerve Fiber Layer (RNFL) at the top should be thick and uniform; thinning is a primary indicator of glaucoma. The central dip, or foveal pit, should have a smooth, concave contour; any distortion can signal problems like an epiretinal membrane. The brightest band at the bottom is the Retinal Pigment Epithelium (RPE), a vital support layer; disruptions here are a hallmark of macular degeneration.
The table below provides a simplified guide to interpreting these key features, linking their appearance on a scan to their anatomical structure and clinical importance. This level of diagnostic granularity is what allows for incredibly early diagnosis and management of conditions like glaucoma and wet AMD, where a few weeks can make a difference in preserving sight.
| Layer Appearance | Anatomical Structure | Clinical Significance |
|---|---|---|
| Top bright layer | RNFL (Retinal Nerve Fiber Layer) | Thinning indicates glaucoma progression |
| Central depression | Foveal pit | Distortion suggests epiretinal membrane |
| Dark spaces within layers | Fluid accumulation | Indicates diabetic edema or wet AMD |
| Bright outer band | RPE (Retinal Pigment Epithelium) | Support system; disruption indicates AMD |
Photo Documentation or Live Inspection: Why You Need Both?
The debate between Optomap and dilation often mistakenly pits one against the other as a replacement. From a technology consultant’s perspective, this is the wrong framework. They are not competitors but complementary tools, each providing a unique and valuable form of data. Photo documentation (Optomap) offers a static, wide-angle, permanent record, while live inspection (through a dilated pupil) offers a dynamic, stereoscopic, real-time assessment. The optimal diagnostic strategy involves leveraging the strengths of both.
A digital retinal image serves as an objective, unchangeable baseline. This temporal record is invaluable for tracking subtle changes over years, such as the slow growth of a nevus (a mole in the eye) or the progression of diabetic retinopathy. It allows for precise, side-by-side comparisons that are impossible with hand-drawn notes from a live exam. Furthermore, these digital images are ideal for telemedicine consultations and can be analyzed by AI algorithms to screen for disease. Conversely, a live, dilated exam provides a true 3D view, allowing the doctor to appreciate the elevation of the optic nerve or the subtle traction on the retinal surface. The doctor can also look at the extreme periphery by having the patient move their eye, sometimes reaching areas even a wide-field camera cannot.
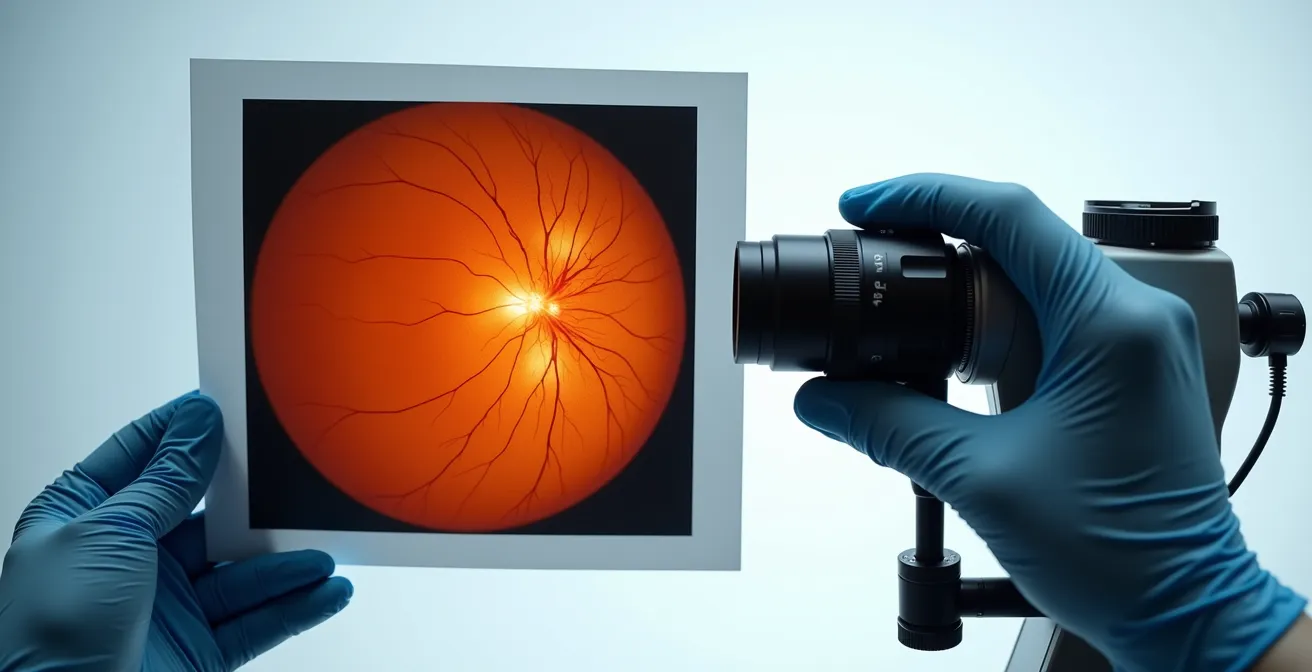
Evidence shows that a combined approach yields superior results. A 2014 study found a 30% improvement in lesion detection when combining optomap with clinical examination. This synergy is critical for ensuring nothing is missed. As the experts at the Eye and Brain Journal noted when evaluating disagreements between methods:
The adjudicator agreed with the image-assisted method in over 70% of cases when methods disagreed.
– Brown, Sewell, Trempe, et al., Eye and Brain Journal
This highlights the immense value of having an objective image to reference. The most comprehensive exam protocol, therefore, is not a choice between the two but an integration of both, tailored to the patient’s individual risk profile.
The Myth That Retinal Cameras Can Damage Your Vision With Bright Light
A common concern for patients considering advanced retinal imaging is the safety of the bright flash of light used to capture the image. It’s a logical question: can this intense light harm the very structures it’s designed to inspect? The answer, based on extensive safety data and international standards, is a definitive no. The technology is engineered with multiple safety mechanisms to ensure the light exposure is well within safe and comfortable limits for the human eye.
The flash from a device like an Optomap is incredibly brief, lasting only a fraction of a second (milliseconds). The critical factor is not the brightness of the flash, but the total light energy delivered to the retina. This total energy is strictly regulated and falls far below the thresholds established by international safety standards, specifically ISO 15004-2 for ophthalmic instruments. To put it in perspective, the total light energy from a single Optomap scan is less than what the retina absorbs in 30 to 60 seconds of looking at a clear blue sky. The system also uses specific filters to remove any potentially harmful invisible wavelengths, such as UV light, using only the necessary spectrum for a high-quality diagnostic image.
The most compelling evidence for the technology’s safety is its extensive track record. The technology has been used for millions of examinations worldwide without incident. According to data compiled on the technology’s use, no adverse health effects have been reported in over 150 million sessions. This demonstrates an exceptional safety profile. The brief after-image or dazzling sensation experienced by some patients is a normal physiological response to a bright light stimulus and fades within seconds, with no lasting effect on vision or retinal health. Patients can be confident that they are undergoing a safe, highly regulated, and non-invasive procedure.
When to Rush In: Sudden Flashes vs. Old Floaters
The vitreous cavity of the eye is not perfectly clear; it contains microscopic collagen fibers that can clump together and cast shadows on the retina. These are “floaters,” and most are harmless, long-standing, and change little over time. However, a sudden change in their appearance or the new onset of light flashes can be a sign of a serious event, such as a retinal tear or detachment, that requires immediate medical attention. Distinguishing between a benign floater and a true emergency is a critical piece of health literacy for every adult.
Flashes of light, often described as lightning streaks in the peripheral vision, are caused by mechanical stimulation of the retina. When the vitreous gel pulls or tugs on the retina, the retinal cells fire, and the brain interprets this signal as a flash of light. While occasional flashes can occur, a sudden onset of new, persistent flashes is a significant warning sign. Similarly, a “shower” of new black dots or a sudden, dramatic increase in the number or size of floaters can indicate bleeding or pigment release from a retinal tear. The most alarming symptom is a curtain or shadow appearing in your field of vision, which may signal that a detachment is already in progress.
The urgency of these symptoms is backed by data. A 2024 prospective study published in *Eye* revealed that 9.9% of patients presenting with acute PVD symptoms (flashes and floaters) were found to have a retinal tear at their initial examination. This statistic highlights that nearly one in ten people with these new symptoms has a condition that could lead to vision loss if not treated promptly. Waiting to “see if it gets better” is a dangerous gamble.
Your Action Plan: Emergency Symptoms Requiring Immediate Evaluation
- Observe for a sudden shower of new black dots or cobwebs (potential blood/pigment from a tear).
- Note any sharp, lightning-like flashes in your peripheral vision (mechanical retinal stimulation).
- Be aware of any progressive curtain or shadow in your visual field (possible detachment in progress).
- Monitor for a sudden and significant increase in floater quantity or size within a 24-48 hour period.
- Contact your eye doctor or go to an emergency room immediately if you experience any of these symptoms.
How Retinal Imaging Replaces the Ophthalmoscope in Modern Exams?
For over 150 years, the direct ophthalmoscope was the primary tool for viewing the retina. This handheld device provides a magnified, but very narrow, “keyhole” view of the fundus. While revolutionary for its time, its limitations are significant in the modern era of data-driven medicine. A complete retinal exam with an ophthalmoscope requires the clinician to mentally stitch together dozens of small, circular views to form a composite picture of the retina—a process that is highly dependent on operator skill and patient cooperation. Crucially, it produces no permanent record beyond the clinician’s hand-drawn notes.
Modern retinal imaging technologies, such as Optomap, represent a fundamental paradigm shift from subjective inspection to objective documentation. Instead of a 10-15 degree keyhole view, a single digital capture can document a 200-degree field of view, covering 82% of the retina. This eliminates the risk of missing pathology that might lie between the small, overlapping views of an ophthalmoscope. The process is standardized, producing a high-resolution, repeatable image that is not dependent on the operator’s drawing skills or memory.
This technological leap has profound implications for quality of care. The digital image creates that vital temporal record for long-term disease management. It enables telemedicine, allowing a specialist hundreds of miles away to review the image instantly. It also opens the door for powerful AI-driven analysis to screen for conditions like diabetic retinopathy. The following table provides a direct comparison of the operational capabilities of these two technologies.
| Feature | Direct Ophthalmoscope | Optomap Imaging |
|---|---|---|
| Field of View | 10-15 degrees | 200 degrees (82% of retina) |
| Documentation | Hand-drawn notes | Digital image storage |
| Operator Dependency | Highly skill-dependent | Standardized capture |
| Telemedicine Capability | None | Instant remote consultation |
| AI Integration | Not possible | Automated screening available |
The Silent Retinal Issue That Standard Acuity Tests Miss in 1 out of 5 Patients
One of the most dangerous misconceptions about vision is that the ability to read the 20/20 line on an eye chart equates to having healthy eyes. This is false. Standard acuity tests measure only the function of the macula, the tiny central part of the retina responsible for sharp, detailed vision. They are completely insensitive to the loss of peripheral vision, which is the hallmark of glaucoma, a leading cause of irreversible blindness.
Glaucoma is often called the “sneak thief of sight” for this very reason. It typically damages the optic nerve fibers that correspond to your peripheral vision first. This loss is so gradual and occurs so far out in the periphery that the brain compensates, filling in the missing information. You don’t notice the loss until the damage is severe and has encroached upon your central vision, at which point it is too late. An individual can have advanced, legally-blinding peripheral field loss from glaucoma while still reading the 20/20 line perfectly. According to 2024 JAMA Ophthalmology research, nearly 4.22 million Americans have glaucoma, with an estimated 50% of them completely unaware they have the condition.

This is another area where a comprehensive retinal examination that includes wide-field imaging and optic nerve analysis (often with OCT) becomes critical. An Optomap can reveal subtle changes to the optic nerve head or thinning of the nerve fiber layer in the periphery, which are early signs of glaucomatous damage. Dilation allows the doctor to get a 3D view of the optic nerve to assess its structure. Relying solely on a visual acuity test and a quick, undilated check is insufficient for screening for this silent disease. It is a perfect example of a condition that requires looking beyond the “what” (acuity) to the “how” (structural integrity of the entire retina and optic nerve).
Key Takeaways
- The debate between Optomap and dilation is primarily about the type and purpose of diagnostic information, not just patient comfort.
- Ultra-widefield imaging is unparalleled for documenting a permanent, wide-angle record of the retinal surface, crucial for monitoring peripheral health and tracking changes over time.
- Combining technologies—using imaging for broad surveillance and a permanent record, and dilation for targeted, 3D assessment—provides the most comprehensive diagnostic yield.
Why Fluctuating Blood Sugar Causes Blurry Vision Even With New Glasses?
For individuals with diabetes, vision that blurs and clears intermittently can be a frustrating and confusing experience, especially when a brand new pair of glasses doesn’t seem to solve the problem. This phenomenon is often a direct result of fluctuating blood glucose levels and is not related to diabetic retinopathy, the more permanent vessel damage associated with long-term diabetes. Instead, this temporary blur is a functional issue caused by changes within the eye’s natural lens.
The lens of the eye is responsible for fine-tuning focus, much like the lens of a camera. When blood sugar levels are high, this excess sugar creates an osmotic gradient that pulls fluid from other parts of the eye into the lens. This influx of fluid causes the lens to physically swell and change shape. This swelling alters its focusing power, typically inducing a “myopic shift,” which means the eye becomes temporarily more nearsighted. This is why vision becomes blurry, and it’s why an old prescription might suddenly seem to work better, or a new one feels completely wrong.
The key takeaway is that this type of blur is reversible. As blood sugar levels are brought back into the target range, the fluid is drawn out of the lens, it returns to its original shape, and vision clears. It is critical for diabetic patients to understand this mechanism. Chasing these fluctuations with new glasses prescriptions is futile and expensive; the solution is not new lenses, but better glycemic control. This phenomenon is a powerful example of how the eye acts as a real-time systemic biomarker, providing immediate feedback on the body’s overall metabolic state. A comprehensive retinal exam in a diabetic patient is therefore about more than just checking for retinopathy; it’s about evaluating the entire ocular system in the context of their systemic health.
Ultimately, the best retinal exam is one that is tailored to your specific health profile and risk factors. The conversation with your doctor should move beyond comfort to focus on diagnostic strategy. By understanding the unique data each technology provides, you can become an active partner in preserving not just your vision, but your overall health.
Frequently Asked Questions about Optomap or Dilation: Which Retinal Exam Is Right for You?
Is the Optomap flash harmful to my retina?
No, the flash is extremely brief (milliseconds) and delivers total light energy far below international safety thresholds (ISO 15004-2). The technology is designed to be completely safe for the eye.
How does the Optomap flash compare to natural sunlight?
The total energy from one Optomap flash is less than what your retina absorbs in 30-60 seconds of looking at a blue sky, making its impact negligible.
Has anyone been harmed by Optomap imaging?
No adverse health effects have been reported in over 150 million Optomap sessions worldwide, demonstrating an exceptional and proven safety profile.
Why does my vision blur when my blood sugar is high?
High blood sugar draws fluid into the eye’s natural lens, causing it to swell and change its focusing power. This physical change induces a temporary myopic (nearsighted) shift, resulting in blurry vision.
Is this blurry vision permanent?
No, it’s reversible. As blood sugar stabilizes and returns to a normal range, the excess fluid leaves the lens, it returns to its original shape, and vision typically clears without any lasting damage.
How is this different from diabetic retinopathy?
Lens swelling causes a temporary, functional blur due to changes in focusing power. Diabetic retinopathy is a permanent, structural disease involving damage to the blood vessels of the retina itself, which can lead to irreversible vision loss.