
The debate over peroxide versus multipurpose solutions misses the critical point. True safety for your eyes isn’t determined by the solution in the bottle, but by your ability to master the specific routine required to defeat microbial threats. This guide reveals that the most significant risks—from stubborn biofilms to neutralization failures—stem from procedural errors, not the chemical’s inherent efficacy.
For any hygiene-conscious contact lens wearer, the choice between a hydrogen peroxide system and a multipurpose solution can feel like a high-stakes decision. One is lauded for its superior disinfecting power, while the other offers unparalleled convenience. The internet is filled with basic advice: peroxide is stronger but must be neutralized, while multipurpose solutions are simpler but may be less effective. This surface-level comparison, however, often overlooks the fundamental truth of ocular hygiene.
The real question is not simply “which is better?” but “which system’s failure points do I best understand and can most reliably avoid?” True sterilization mastery lies in understanding the unseen enemy: pathogens like bacteria and amoebas, and the resilient structures they form, such as bacterial biofilms. Safety is not a feature of the product; it is the outcome of a flawless process. A lapse in aseptic technique can render even the most potent disinfectant ineffective and turn a simple convenience into a source of infection.
This article moves beyond the marketing claims to provide a scientific and decisive analysis. We will deconstruct the microbiological vulnerabilities of each system, exposing the common procedural errors that lead to contamination and eye infections. By understanding the *why* behind each step of the cleaning routine, you can make an informed choice and, more importantly, master the protocol required to ensure your eyes remain truly safe.
This comprehensive guide will explore the critical aspects of contact lens hygiene, breaking down the science behind each potential risk and providing clear, actionable protocols. The following sections will equip you with the expert knowledge needed to manage your lens care routine with clinical precision.
Summary: Peroxide vs. Multipurpose: A Sterilization Expert’s Guide to Eye Safety
- Why Rinsing Cases With Tap Water Risks Acanthamoeba Infection?
- How to Perform the “Palm Rub” Technique Without Tearing the Lens?
- Chemical Disinfection or Daily Disposables: Which Is Better for Germaphobes?
- The Bacterial Growth Caused by Adding Fresh Solution to Old Fluid
- Problem & Solution: Avoiding Chemical Burns With Peroxide Systems
- How to Disinfect Pillowcases and Towels to Prevent Pink Eye Reinfection?
- Why Plastic Cases Harbor Bacteria Even After Rinsing?
- Why Soaking Alone Fails to Remove Stubborn Bacterial Biofilms?
Why Rinsing Cases With Tap Water Risks Acanthamoeba Infection?
Using tap water to rinse contact lenses or their case is a critical and dangerous error in hygiene protocol. While it may seem harmless, tap water is not sterile and can harbor a variety of microorganisms, the most notorious of which is Acanthamoeba. This free-living amoeba is ubiquitous in water sources, including lakes, oceans, and municipal tap water. For most people, exposure is harmless. For a contact lens wearer, it can lead to a devastating and painful corneal infection.
The danger lies in the organism’s unique biology. Acanthamoeba exists in two forms: an active, feeding trophozoite and a dormant, highly resistant cyst. As the CDC’s Morbidity and Mortality Weekly Report states, “Because of its ability to encyst in extreme environmental conditions, the organism is difficult to kill.” When a lens or case is rinsed with contaminated water, these cysts can attach. Once on the eye, they can revert to their active form, invade the cornea, and cause Acanthamoeba keratitis (AK), an infection that can lead to severe pain and blindness.
The risk is not theoretical. Data from a 2019 CDC report on an AK outbreak in Iowa is stark: among the affected contact lens wearers, over 85% reported inadequate hygiene practices. These practices included showering while wearing contacts, swimming in lakes, and, critically, cleaning contacts with tap water. While the overall incidence of AK is low—affecting one to two new cases per 1 million contact lens wearers annually—the consequences are severe, and the cause is almost always preventable exposure to non-sterile water.
Therefore, the directive is absolute: never allow tap water, or any non-sterile liquid, to come into contact with your contact lenses or storage case. Only use commercially prepared, sterile solutions designed for contact lens care.
How to Perform the “Palm Rub” Technique Without Tearing the Lens?
Mechanical cleaning—the “rub and rinse” step—is a non-negotiable part of any disinfection routine for reusable lenses, even when using “no-rub” labeled solutions. This physical friction is essential for dislodging protein deposits, lipids, and debris that accumulate on the lens surface. More importantly, it helps to break apart the initial formation of microbial biofilms that chemical soaking alone may not penetrate. Performing this technique correctly is paramount to ensure the lens is clean without causing damage.
The key to a safe “palm rub” is using the right pressure and tools: your fingertips, not your fingernails. A soft contact lens is a delicate piece of medical-grade hydrogel, and aggressive handling can easily cause microscopic tears or a full rip. The goal is gentle, consistent friction. The pad of your finger provides a soft, broad surface that can effectively clean the lens without concentrating pressure on a single point.
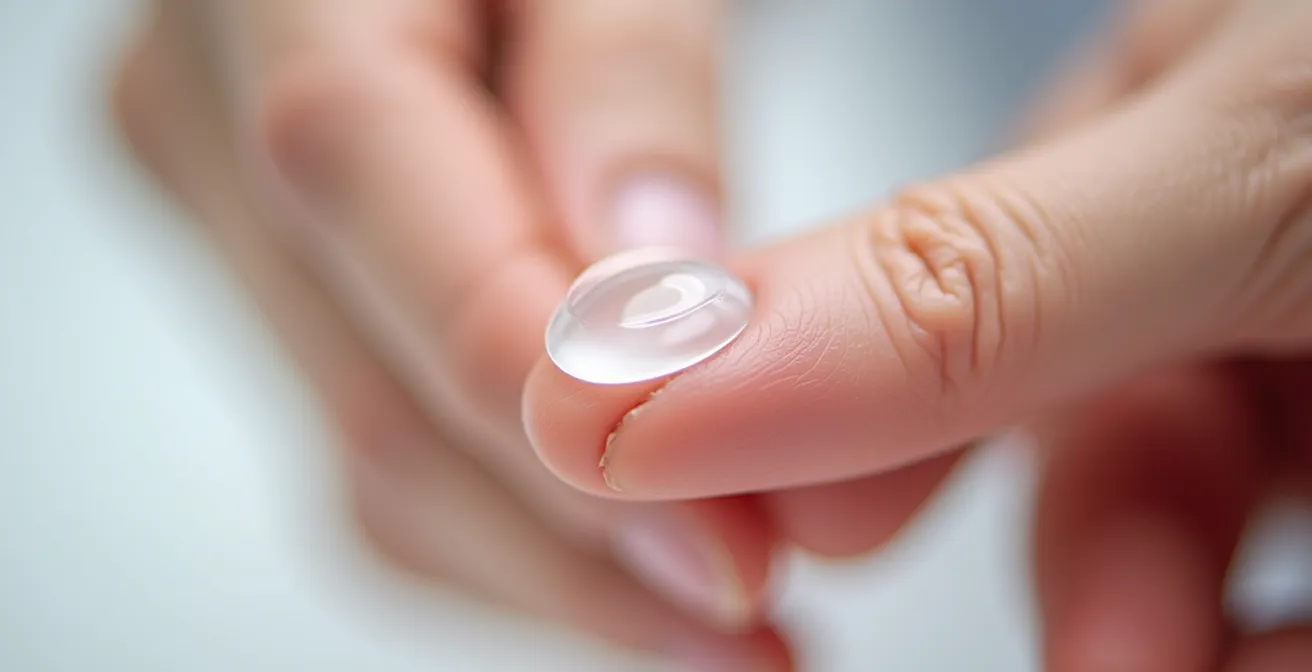
The procedure is a core component of aseptic technique. Before beginning, always wash your hands thoroughly with soap and water, and dry them with a lint-free towel. Place the lens in the clean palm of your hand, add a few drops of fresh multipurpose solution, and use the pad of your index finger to gently rub both sides of the lens in a back-and-forth motion for approximately 10-20 seconds. Never use a circular motion, as this can stress the material. Following the rub, thoroughly rinse the lens with the solution to wash away all loosened debris before placing it in the clean case.
This simple, manual step dramatically increases the efficacy of any disinfection system by physically removing the microbial load and deposits before the chemical disinfection phase even begins.
Chemical Disinfection or Daily Disposables: Which Is Better for Germaphobes?
For the user most concerned with eliminating pathogens—the “germaphobe”—the choice of lens modality and disinfection system is critical. The debate often centers on the efficacy of hydrogen peroxide versus multipurpose solutions, but the ultimate solution may be to eliminate the need for cleaning altogether. From a purely microbiological standpoint, daily disposable lenses are the safest option available. They eliminate the risks associated with cleaning, storage cases, and solution sensitivity, as a fresh, sterile lens is used each day.
The data supports this conclusion unequivocally. A 2022 case-control study revealed that the risk for Acanthamoeba keratitis is more than three times higher in users of reusable lenses compared to those who use daily disposables. This is because daily disposables remove the primary vectors of contamination: the lens case and the human element of a cleaning regimen.
However, for those who use reusable lenses, the choice between disinfection systems matters. Hydrogen peroxide solutions are generally considered the gold standard for disinfection. They are preservative-free and offer superior antimicrobial efficacy, particularly against stubborn organisms and biofilms. Their primary drawback is the absolute requirement for a neutralization step. In contrast, multipurpose solutions offer convenience by combining cleaning, rinsing, disinfecting, and storing into one product, but they contain preservatives that can cause sensitivity in some users and may be less effective against certain resilient pathogens.
The following table, based on information from the American Academy of Ophthalmology, breaks down the key differences:
| Feature | Hydrogen Peroxide | Multipurpose Solution |
|---|---|---|
| Preservatives | Preservative-free | Contains preservatives |
| Disinfection Time | 6-8 hours minimum | 4-6 hours |
| Cost | More expensive | Less expensive |
| Effectiveness | Superior disinfection | Good disinfection |
| Risk if misused | Severe burning/damage | Mild irritation |
For the true germaphobe, the hierarchy is clear: daily disposables are superior. If using reusable lenses, a hydrogen peroxide system offers the highest level of chemical disinfection, provided its strict usage protocol is followed without exception.
The Bacterial Growth Caused by Adding Fresh Solution to Old Fluid
The practice of “topping off”—adding fresh contact lens solution to the used solution already in the case—is a common but extremely hazardous shortcut. It fundamentally compromises the entire disinfection process by diluting the active ingredients of the fresh solution and, more alarmingly, creating an ideal environment for microbial proliferation. This seemingly innocent act effectively turns the lens case into an incubator for bacteria.
Disinfecting solutions are formulated with a specific concentration of antimicrobial agents designed to kill a certain level of microbial load within a set time frame. When you “top off,” the old solution is no longer at its full strength; its disinfecting power has been depleted, and it is contaminated with debris and microorganisms from the previous wear. Mixing it with fresh fluid reduces the overall concentration of the active ingredients below the effective threshold. The CDC is explicit in its warning: “Avoid ‘topping off’ by never mixing fresh solution with old or used solution in the case, as it reduces disinfection effectiveness.”

This compromised solution is not only less effective at killing new pathogens introduced by the lens, but it also allows the surviving microorganisms from the previous day to thrive. The result is a rapid increase in the bacterial population within the case. This practice is a significant risk factor for serious eye infections. In fact, research shows a nearly 5-fold increased risk of microbial keratitis for wearers who top off their solution compared to those who use fresh solution every time. It directly contributes to the formation of dangerous biofilms on both the lens and the case itself.
The correct procedure is absolute: after each use, empty the case completely, rinse it with fresh sterile solution (never water), let it air dry, and only then refill it with fresh solution for the next disinfection cycle.
Problem & Solution: Avoiding Chemical Burns With Peroxide Systems
The primary risk associated with hydrogen peroxide systems is not a failure of disinfection, but a failure of neutralization. Hydrogen peroxide at a 3% concentration is a powerful oxidizing agent, highly effective at destroying pathogens. However, this same chemical power makes it extremely caustic to the delicate tissues of the cornea. A chemical burn to the eye from unneutralized peroxide is intensely painful and can cause significant, though usually temporary, damage. Preventing this is the central challenge of using a peroxide system.
The Problem: Neutralization Failure
Neutralization failure occurs for two main reasons. The first is user error: a wearer might forget the system requires neutralization and attempt to rinse a lens with the peroxide solution or put a lens directly into their eye from the soaking basket before the process is complete. The second, more subtle reason, is a failure of the neutralization mechanism itself. Most modern peroxide systems use a special case containing a platinum-coated disc. This disc acts as a catalyst, breaking down the hydrogen peroxide (H2O2) into harmless water (H2O) and oxygen (O2). This process is not instantaneous; it requires a minimum soaking time of 6 to 8 hours.
Interrupting this process prematurely means the solution still contains a significant concentration of active peroxide. Furthermore, the catalytic disc itself has a limited lifespan. Reusing an old case beyond the manufacturer’s recommendation (typically 1-3 months) means the catalyst may be depleted and unable to fully neutralize the peroxide, even if the full soaking time is observed. This is a critical point often overlooked by users.
The Solution: Strict Adherence to Protocol
The solution is a rigid, unwavering adherence to the manufacturer’s instructions. First, always use the special case that comes with the peroxide solution; never use a standard flat case, as it lacks the neutralizing catalyst. Second, respect the minimum 6-hour soak time without exception. Set a timer if necessary. Third, and critically, discard the case and replace it with the new one provided in each new box of solution. This ensures your catalytic disc is always effective. By internalizing this protocol, the risk of a chemical burn is virtually eliminated, allowing the user to benefit from peroxide’s superior disinfecting power safely.
How to Disinfect Pillowcases and Towels to Prevent Pink Eye Reinfection?
Effective ocular hygiene extends beyond the contact lenses themselves and into the immediate environment. When dealing with an active eye infection like conjunctivitis (pink eye) or recovering from one, preventing reinfection is paramount. Everyday items that come into contact with the face, particularly porous materials like towels and bedding, can act as fomites—objects that harbor and transmit pathogens. A thorough decontamination of these items is a critical step in breaking the cycle of infection.
The primary pathogens responsible for conjunctivitis can survive on surfaces for extended periods. Bacterial causes may persist for several hours, while some viruses can remain viable for up to two days. Therefore, a simple wash may not be sufficient. The key to effective disinfection is using a combination of hot water and high heat drying. Most bacteria and viruses are killed at temperatures above 140°F (60°C). Washing pillowcases, towels, and any other cloths that have touched the face in hot water is the first line of defense.
For an added layer of security, especially with viral or aggressive bacterial infections, using a laundry sanitizer or an oxygen-based bleach product in the wash can further reduce the microbial load. Once washed, items must be dried on the highest heat setting available. The sustained high temperature of a clothes dryer is highly effective at killing any remaining pathogens. This protocol should be followed for all personal items until at least 24-48 hours after symptoms have completely resolved.
Action Plan: Household Decontamination for Eye Infections
- Wash all bedding and towels in hot water (at least 140°F/60°C).
- Use an oxygen-based bleach or laundry sanitizer for extra disinfection during the wash cycle.
- Dry all items on a high heat setting to kill any remaining pathogens.
- Replace or thoroughly disinfect any eyeglasses and sunglasses worn during the infection.
- Clean and disinfect phone screens, remote controls, and any other surfaces that regularly touch the face or hands.
- Discard all eye makeup (mascara, eyeliner) and applicators used during or immediately before the infection.
By expanding the field of hygiene from just the lenses to the entire personal environment, you adopt the thorough mindset of a sterilization expert, leaving no room for pathogens to persist.
Key Takeaways
- True contact lens safety lies in mastering the cleaning process, not just choosing a product.
- Biofilms are the primary enemy, requiring both physical rubbing and potent chemical disinfection to be defeated.
- Human error, such as topping off solution or using tap water, is a far greater risk than the inherent limitations of any given solution.
Why Plastic Cases Harbor Bacteria Even After Rinsing?
The contact lens case is arguably the most critical and most frequently neglected component of the entire hygiene system. Many users believe a quick rinse is sufficient to keep it clean, but this assumption is dangerously flawed. The plastic material of a standard lens case, with its microscopic grooves and crevices, is a perfect breeding ground for bacteria and the formation of a resilient, invisible slime layer known as a biofilm.
Not replacing your case can cause the formation of a biofilm that most solutions cannot disinfect.
– Moran CORE, University of Utah
A biofilm is a community of microorganisms encased in a self-produced protective matrix. This matrix acts like a shield, making the bacteria within it hundreds of times more resistant to disinfectants than free-floating bacteria. Once a biofilm is established inside a lens case, a simple rinse with solution is utterly ineffective at removing it. Even vigorous rubbing may not be enough to dislodge these entrenched colonies. The lenses then sit in this “bacterial city” overnight, emerging contaminated each morning.
This risk is amplified exponentially if improper fluids are ever introduced. For instance, storing lenses in non-sterile fluids is a catastrophic error; studies show a 16-fold increase in infection risk when lenses are stored in tap water, which directly inoculates the case with biofilm-forming organisms. The only effective strategy for managing this risk is a combination of proper daily care and, most importantly, frequent replacement. The daily routine should involve emptying the old solution, rinsing the case with fresh sterile solution, and allowing it to air-dry upside down on a clean surface. However, even with perfect care, biofilms will eventually begin to form.
For this reason, all major health organizations and manufacturers recommend replacing the contact lens case at least every three months, if not monthly. This simple, low-cost habit is one of the most effective measures you can take to prevent serious eye infections.
Why Soaking Alone Fails to Remove Stubborn Bacterial Biofilms?
The belief that simply soaking contact lenses in solution is sufficient for disinfection is one of the most pervasive and dangerous misconceptions in lens care. This passive approach ignores the primary challenge in microbial control: the bacterial biofilm. As previously discussed, a biofilm is not just a collection of germs; it is a structured, fortified community. Soaking a lens coated in a developing biofilm is like trying to wash a car by letting it sit in a puddle—it’s fundamentally ineffective.
The protective slimy matrix of a biofilm prevents the disinfecting chemicals in the solution from reaching the bacteria within. Multipurpose solutions, in particular, may struggle to penetrate a mature biofilm. This is precisely why the “rub and rinse” step is non-negotiable. The physical friction from rubbing is required to mechanically disrupt and break apart this protective layer, exposing the underlying bacteria to the chemical action of the disinfectant. Without this step, you are merely disinfecting the outer layer of the biofilm, leaving the core community intact and ready to regrow.
This is also where the chemical nature of the disinfectant becomes critical. Hydrogen peroxide systems have a distinct advantage in this area. According to scientific understanding of their properties, “Hydrogen peroxide has the ability to penetrate microbial films, which helps create a deeper clean.” The strong oxidizing action of peroxide can break down the biofilm matrix more effectively than many of the gentler agents found in multipurpose solutions. The bubbling action you see when peroxide is neutralized by its catalytic disc also provides a form of micro-agitation, further helping to lift and remove debris.
Therefore, the complete, scientifically-backed disinfection protocol involves a synergistic attack: first, a manual “rub” to break the biofilm’s defenses, followed by a thorough “rinse” to remove the debris, and finally, a chemical “soak” to kill the exposed and remaining pathogens. Skipping any of these steps leaves your eyes vulnerable to infection.
Frequent questions on Peroxide or Multipurpose: Which Disinfection System Is Safer for Your Eyes?
How long can pink eye bacteria survive on surfaces?
Bacterial conjunctivitis pathogens can survive on surfaces for 2-8 hours, while viral causes can persist for up to 48 hours on hard surfaces.
When is it safe to resume normal laundry habits after pink eye?
Continue enhanced cleaning protocols for at least 24-48 hours after symptoms resolve and antibiotic treatment ends to prevent reinfection.
Should I throw away my contact lenses after pink eye?
Yes, it is imperative to discard any contact lenses worn during or immediately preceding the infection. Start with a fresh, sterile pair only after you have been cleared by your eye doctor.